Market Access | Reimbursement | Evidence
Your Market Access Repository
Market Access | Reimbursement | Evidence
Your Market Access Repository
Scroll for more
Market Access Radar (MAR) provides quick and easy access to the most important market access (MA)-related information. MAR insights display in an easy-to-follow layout and present the most relevant data directly to you when you need it. Our MAR team gathers the latest reimbursement recommendations and information on financing from public funds announced by Ministries of Health and HTA agencies. This information provides changes in legal provisions regulating financing of health technologies from public funds. In addition, our team gathers the latest news on drug safety, new marketing authorisations, variations in registered and reimbursed indications published by EMA, HMA, FDA, MHRA, Ministries of Health and other national institutions responsible for registration and safety of drugs and other valuable information which may impact reimbursement, published in mass-media and relevant press.
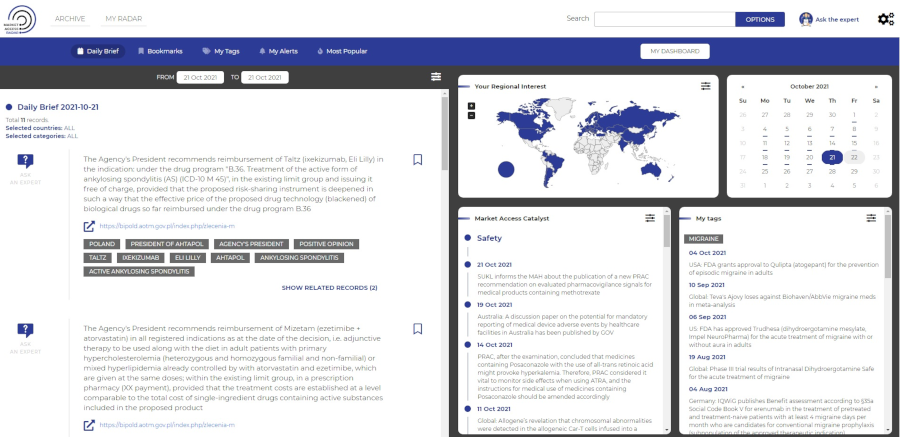
Information created by Experts
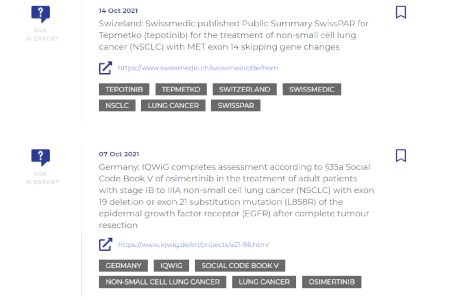
Our team of experts manually assesses and prioritizes the impact of the information, offering decision value for users.
Custom User Profiles
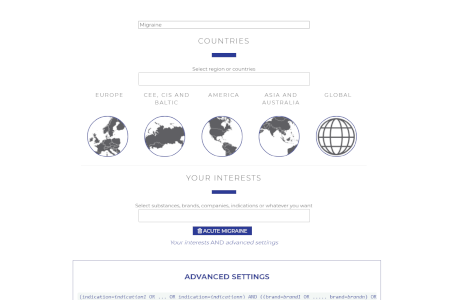
Create different user profiles to tailor your Market Access Repository.
Quick Access
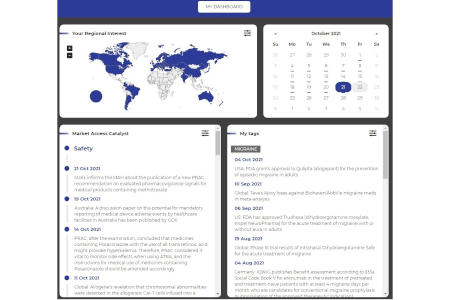
Use My Dashboard or My Tag to get quick access to information from your specified regions or countries of interest.
Ask an Expert
Receive further information on the source by asking your local expert for more information.